 Research
Research
-
Research direction 1: Metal-Free C-H Functionalization via Diaryliodonium Salts with a Chemically Robust Dummy Ligand
Metal-Free C-H Functionalization via Diaryliodonium Salts with a Chemically Robust Dummy Ligand
Release time: 2022-08-11
芳香烃的直接官能化在生物医药、农业、精细化工领域都有着重要应用。向分子的特定位点引入易于转化的基团是结构修饰的重要策略,可以便捷地实现分子官能团化并增加结构多样性。碳氢 (C-H) 键在有机分子中广泛存在,因而碳氢键的快速转化方法得到了越来越广泛关注。然而由于C-H键较为惰性,其活化转化过程常需要过渡金属参与,在无过渡金属存在的条件下实现高选择性C-H键转化仍然是合成领域中的一项重要挑战。 我们团队发展了一类基于3,5-二甲基-4-异噁唑基 (DMIX) 的高价碘试剂。这类试剂易于制备储存,可以通过高位置选择性的C-H官能化反应将芳香化合物快速转化为双芳基碘鎓盐。研究发现DMIX基团具有“不转移”特性,因而可以确保C-C、C-N、C-S及C-O等偶联反应中芳基迁移的选择性。这种两步的合成策略为芳香化合物C-H键选择性修饰提供了一种广泛适用的方法。
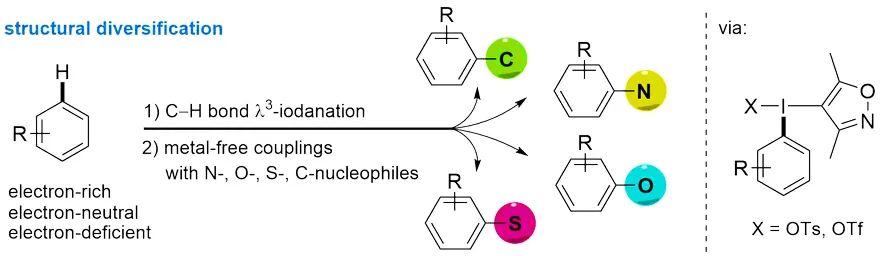
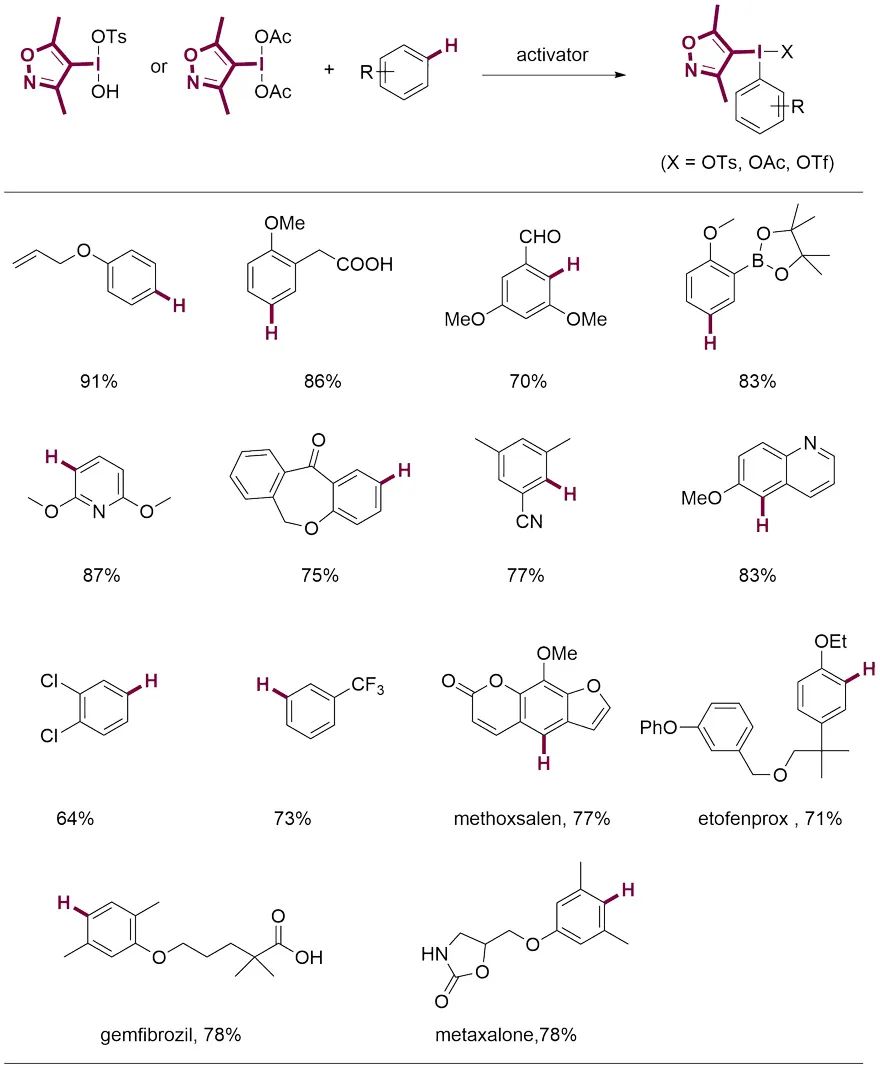
作者从带有DMIX基团的三价碘试剂出发,在室温条件下通过碳氢键官能化以良好至优秀的产率快速制得结构多样的双芳基碘鎓盐。反应能够兼容溴、氰基、酯基、醛基、羧酸和硼酯等活性官能团。醚菊酯、吉非罗齐和美他沙酮等药物分子也能顺利参与反应,说明该反应策略在药物分子后修饰方面具有广阔的应用前景。与其他碳氢C-H键官能化反应相比,新报道方法的独特之处是同时适用于富电子和缺电子芳烃的修饰。
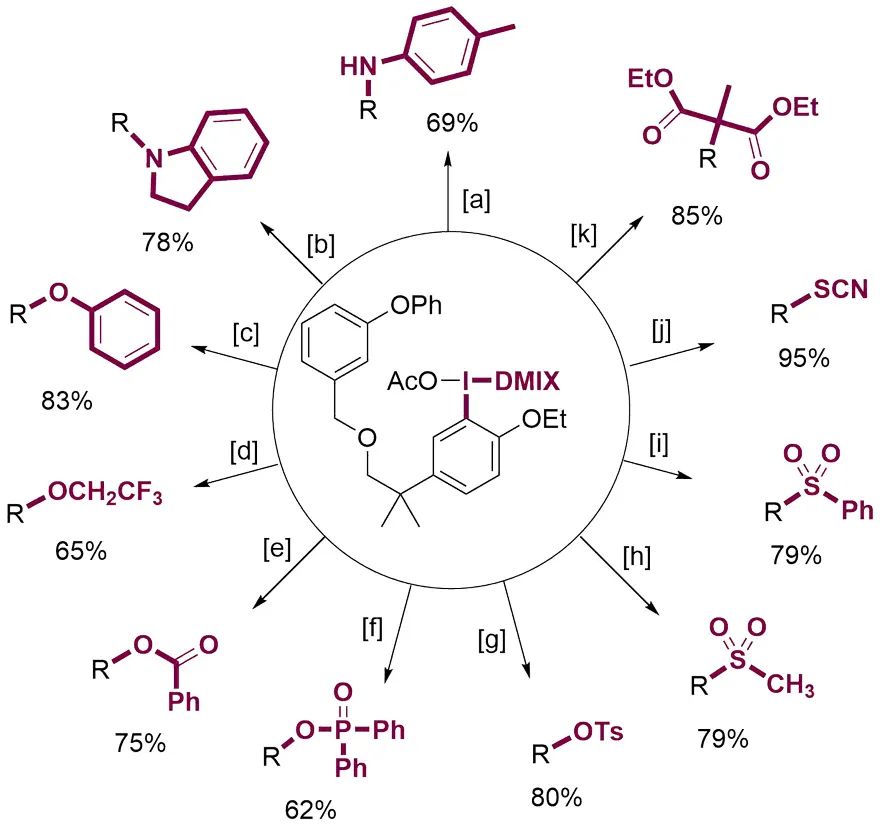
上海有机化学研究所的薛小松团队对DMIX高碘盐在无金属偶联反应中芳基迁移的选择性进行了计算模拟。研究表明,DMIX基团比通常认为不能发生迁移的三甲氧基苯基 (TMP) 更难迁移,因而可以保证后续的偶联反应具有优越的化学选择性。作者以醚菊酯作为示例,通过将带有DMIX基团的碘鎓盐与胺、醇、酚、酸、亚磺酸盐、硫氰酸盐和丙二酸酯等亲核试剂反应,成功在分子中引入各类不同的官能团,实现了醚菊酯的快速多样化修饰。
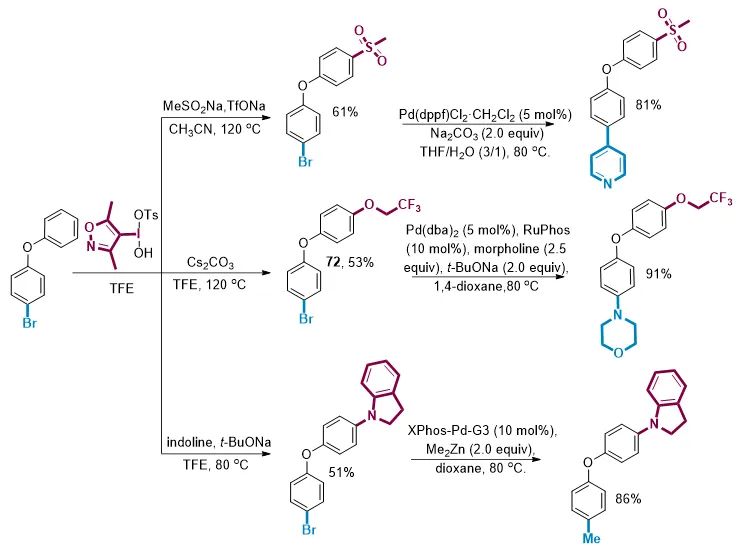
由于这种C-H键官能化反应能够与芳基卤化物兼容,用含溴芳香化合物制备的双芳基高碘盐具有两种能够进行后续转化的基团。通过组合双官能化,就可以迅速增加分子的结构多样性。以4-溴苯基-苯基醚为例,利用高碘盐试剂一锅法就可以实现分子的C-H键官能化,得到C-O、C-S和C-N偶联产物而不破坏C-Br键。结合过渡金属催化对C-Br键进行转化,就可以迅速得到多种不同取代的分子。
综上所述,这项研究为有机分子结构多样化提供了一种新的便捷手段,展示了高价碘化学在介导C-H键官能化反应中的广阔前景。
-
Research direction 2: Recognition-Enabled Automated Analyte Identification Via 19F NMR
Recognition-Enabled Automated Analyte Identification Via 19F NMR
Release time: 2022-08-11
Lewis碱性官能团在药物分子及代谢物中很常见,因此作者选择了三酚配体铝络合物作为研究平台来构建核磁氟谱检测体系。晶体数据库检索表明,这类络合物可以与水及其他溶剂分子在固态下形成加合物,表明其具有合适的络合能力。从苄基保护的水杨醛2出发,经过还原胺化可以得到三级胺3,氢化脱苄基保护即可以得到配体4。配体4与三甲基铝反应可以直接得到结合一分子溶剂的目标络合物1 (THF)(图1)。改变保护的水杨醛2,就可以得到具有不同含氟基团的目标络合物。晶体结构表明络合空腔周围的含氟基团位置很接近分析物,因此细微的结构差别导致的氟原子周围化学环境的变化能够被核磁氟谱所感知。
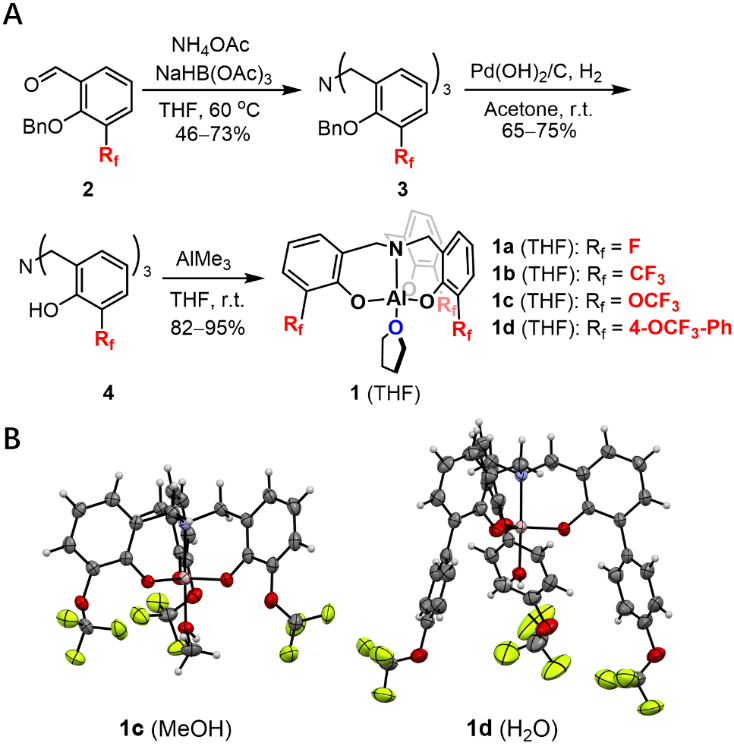
图1 具有不同含氟基团的铝络合物探针的制备
接下来作者选取探针1c,来检验复杂混合体系中对各组分进行识别区分的可行性。将10个具有不同结构和官能团的药物分子混合于氘代氯仿中,采集核磁共振氟谱。如图2所示,在复杂混合物分析中观察到的氟谱核磁信号能够与各个分析物一一对应,并且每个分析物的化学位移与单独检测时的化学位移相同,因此这一探针体系可以进行多组分的原位分析检测,且不需要对样品进行分离。本检测方法中的化学位移与传统色谱学方法中的保留时间类似,可以作为分析物定性检测的特征参数。
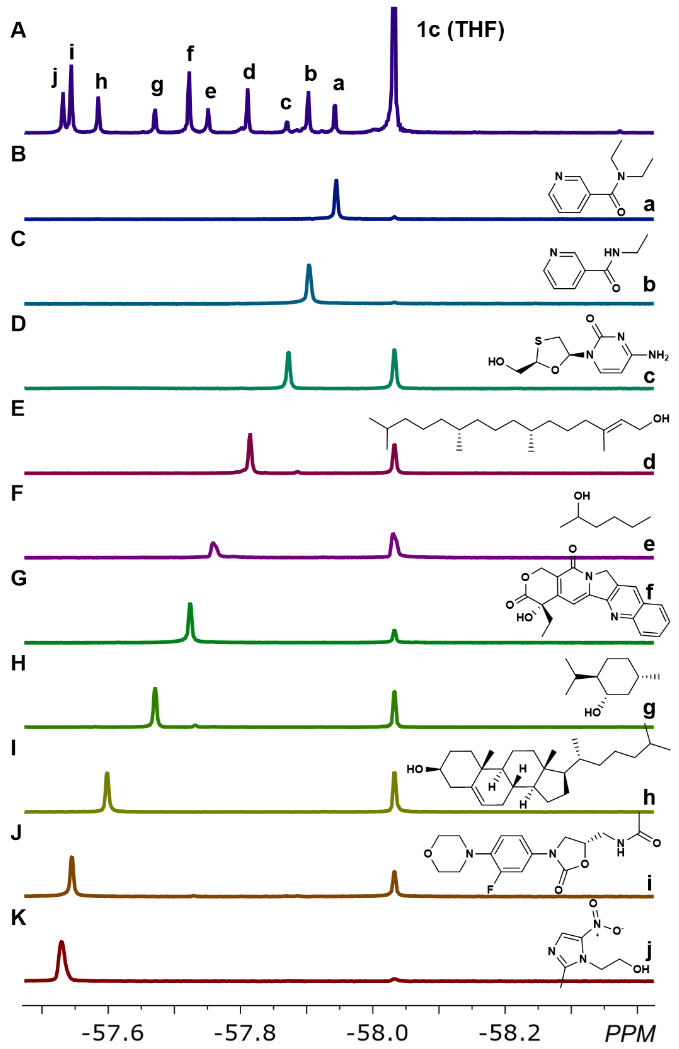
图2 利用探针1c进行多种药物同时分析
食品成分及添加剂的快速检测对食品安全和质量控制至关重要。作者选择对食品中辣味的主要活性成分——辣椒素和二氢辣椒素进行了分析。这两个分析物结构高度类似,却能够用探针1c很好地区分,表明这一探针体系强大的区分能力(图3,E,F)。对泡椒、方便面调味料、干燥的小米椒以及印度魔鬼椒等不同来源样品的分析结果表明,辣椒素和二氢辣椒素都能够被指认出来,说明样品的基质对检测影响很小(图3,A-D)。作为防腐剂的山梨酸以及其他脂肪酸也可以被指认出来,进一步突出了这一方法在原位多组分分析方面的潜力。
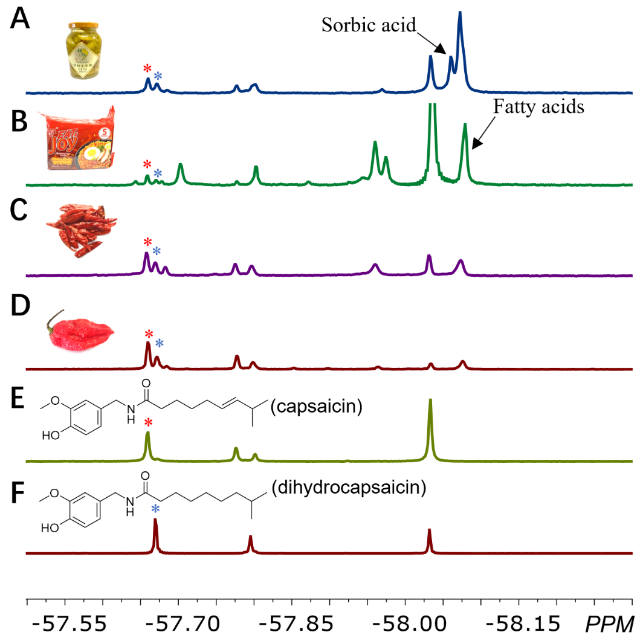
图3 利用探针1c进行辣椒素和二氢辣椒素的区分识别
因为这一氟谱检测体系对每一个分析物都会得到特征且单一的氟谱核磁信号,极大地简化了检测,为分析物的自动识别指认提供了可能。作者接下来用三个探针1b-d检测了三十三中常见的药品,得到了化学位移数据库。使用单独探针进行定性分析时,一些化合物特征氟谱化学比较接近而容易出现误差,因此作者选用了三个探针混合后对未知分析物进行定性分析(图4),利用编程将新得到的核磁信号与数据库中的化学位移进行比对,计算均方根偏差(RMSD,式1),即可对分析物进行指认。对于单一分析物和两种分析物的混合物,这一方法都可以准确指认。随着分析物的增多和数据库的扩大,经过算法优化后,复杂混合物的多组分快速自动化分析将成为可能。
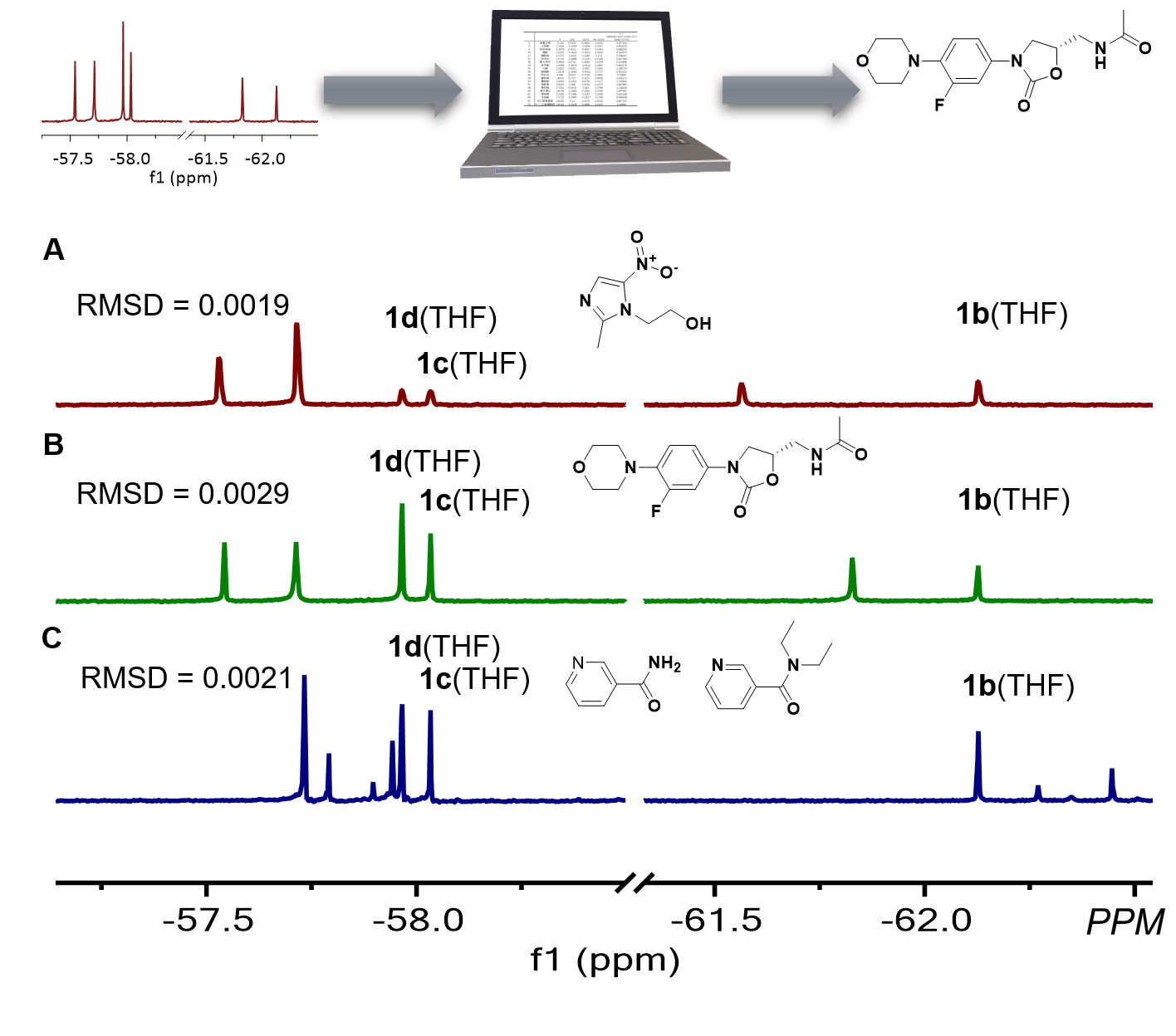 图4 使用探针组合进行自动分析物指认
图4 使用探针组合进行自动分析物指认本文设计了一系列含氟铝络合物探针,可以与含有不同Lewis碱性基团的分析物进行可逆结合。探针与分析物络合之后会产生不随浓度变化的特征核磁氟谱信号,从而可以实现分析物的定性。19F NMR背景信号低,并且基于动态识别特性的探针能够选择性地络合待测物,因此可以大大减少谱图重叠与基质的信号干扰,实现了复杂体系中的分析物指认。利用多探针检测体系,实现了分析物的自动指认。这种基于19F NMR的检测体系能够用于复杂体系检测,分析过程不需要对谱图有深入的理解,且在自动识别指认时有着很高的准确性,因此有望成为一种强大的工具,应用于药物发现、疾病诊断以及质量控制等领域。
-
Research direction 3: Chemical sensing detection method based on nuclear magnetic resonance and secondary hydrogen induced nuclear polarization
Chemical sensing detection method based on nuclear magnetic resonance and secondary hydrogen induced nuclear polarization
Release time: 2020-11-07
Selective detection and identification of analytes plays an important role in environmental detection and biomedical research. The increasing demand for fast and reliable detection methods has promoted the research of various chemical sensors. Usually a molecular recognition process leads to changes in properties such as fluorescence, resistance, and REDOX potential for detection. However, these methods are often easy to be interfered and difficult to distinguish the similar structures to be tested, so they are not suitable for the detection of complex mixtures.
One of the fundamental reasons for these limitations is that small differences in molecular recognition are indistinguishable. Around this scientific problem, probe atoms are distributed around the object to be measured through molecular design. Based on the characteristics that the chemical displacement of some heteroatoms changes significantly with the surrounding environment, nuclear magnetic resonance is used to accurately distinguish similar recognition behaviors. The high sensitivity and accuracy detection method is realized by using nuclear magnetic resonance hyperpolarization method.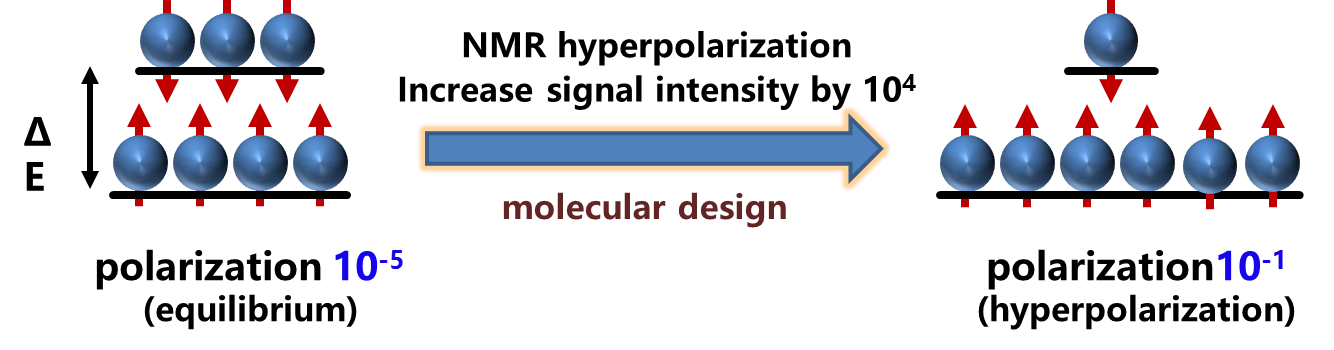
Figure 1 Principle of NMR hyperpolarization method
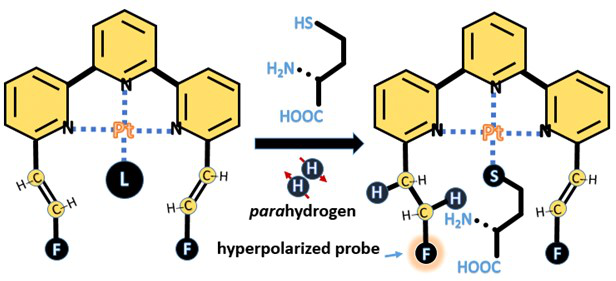
Figure 2 A chemical sensing method based on NUCLEAR magnetic resonance and secondary hydrogen induced nuclear polarization
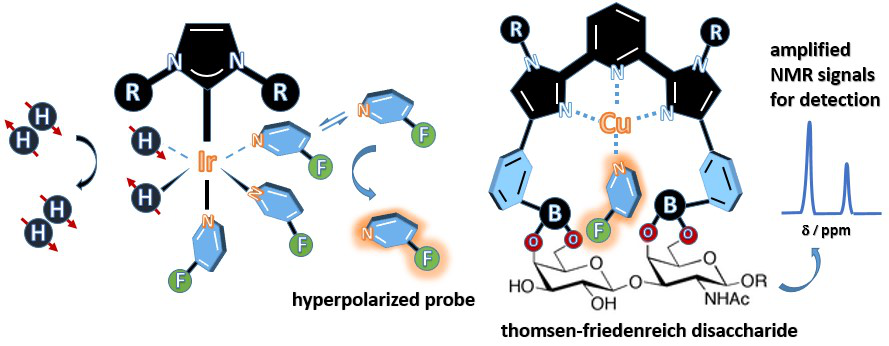
Figure 3 High sensitivity analysis was performed using polarizable probe molecules
-
Research direction 4: Functional fluorinated and porous materials
Functional fluorinated and porous materials
Release time: 2020-09-24
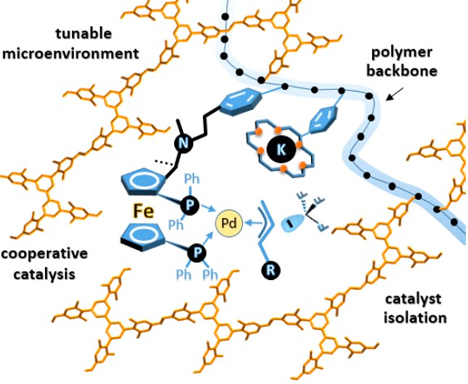
Organic porous materials are used in important applications in the fields of catalysis, gas separation, chemical sensing and environmental protection. However, there are still challenges to synthesize organic porous materials that are easy to process and apply and are highly functionalized. One reason for this limitation is that the synthesis of porous materials is often based on a high degree of cross-linking, forming an insoluble three-dimensional network structure that makes the polymer difficult to process. On the other hand, the disordered polymerization reaction and the limitation of the template increase the difficulty of controlling the micropore structure. We envision the strategy of copolymerizing monomers with large steric hindrance, rigid three-dimensional structure and functionalized monomers based on copolymerization to synthesize highly functionalized one-dimensional linear functionalized organic porous materials. The molecular design of the monomer structure can effectively avoid the close packing of the polymer in the solid phase and promote the formation of pores. At the same time, the one-dimensional linear structure of the material can ensure the easy processing performance of the material. By fine-tuning the microenvironment around the pore size, we will greatly improve the enrichment, separation and catalytic performance of the material.
-
Research direction 5: Mechanochemistry and solid phase organic synthesis
Mechanochemistry and solid phase organic synthesis
Release time: 2020-09-24
Organic chemical reactions in solution generally take place in a disordered state, and the irregular movement of solvent molecules forms a dynamic and chaotic reaction microenvironment around the reaction site, thus affecting the selectivity of various chemical transformations. Efficient reaction control requires precise manipulation of the position, conformation, and direction of the reactants to reduce the activation energy of the required reaction pathway. Manipulation of the reactants in the environment can be greatly simplified if the molecules in the environment come to rest or move more slowly. We will use molecular assembles to create an ordered reaction microenvironment in the solid phase. Through molecular design, some molecules with three-dimensional rigid structure will form ordered molecular containers and molecular channels when they are piled up in solid phase, and the molecules wrapped in these ordered reaction environments can show unique reaction properties of different solution states.
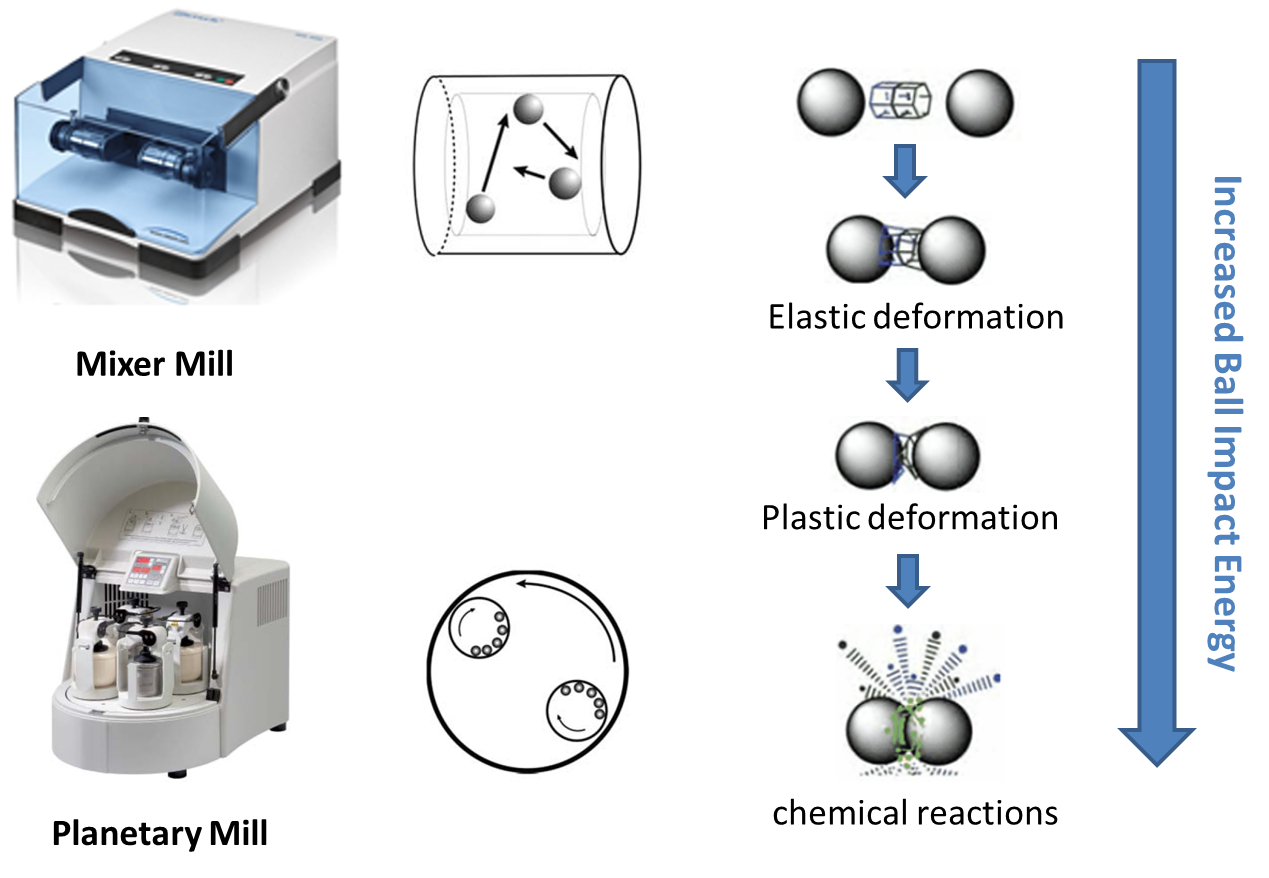
Figure1 Solid phase mechanochemistry
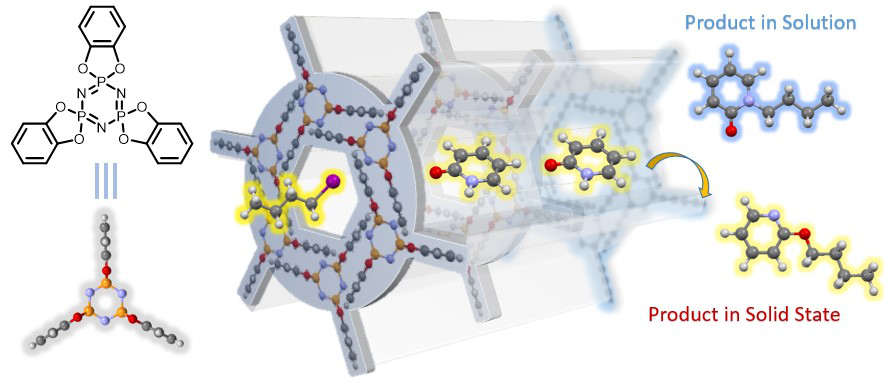
Figure2 Chemical reactions in a solid ordered microenvironment
-

021-54925545
-

zhaoyanchuan@sioc.ac.cn
-

Room 1401, Junmou Building, 345 Lingling Road, Xuhui , Shanghai
Copyright © 2020 Yanchuan Zhao Research group of Key Laboratory of Organic Fluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences All rights reserved
沪ICP备09006577号-3